Description
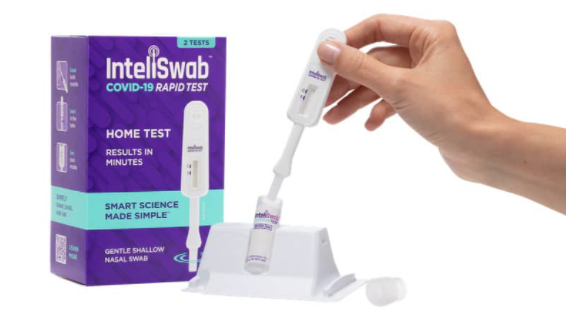
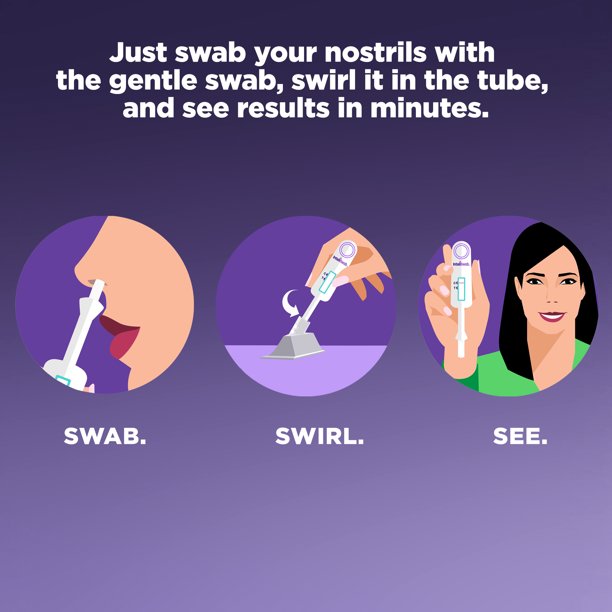
Meet InteliSwab, the COVID-19 rapid antigen test that makes self-testing remarkably simple. It’s so user-friendly, it can be used anytime and anywhere, and requires less than one minute of hands-on time. InteliSwab has received FDA Emergency Use Authorization* for self-testing. You do not need to ship samples to a lab or get a prescription from your healthcare provider. Use requires just 3 key steps: Swab, Swirl and See your result in 30 minutes. There is no assembly required. InteliSwab makes testing so easy, you’ll know you did it right.
- Detects active COVID-19 infection for both symptomatic and asymptomatic use
- Shallow and gentle nasal swab (lower nostril)
- 98% of untrained, unproctored users found InteliSwab™ easy to use
- Requires less than one minute of hands-on time
- No lab needed and no assembly required
- Get your results in 30 minutes
- No difficult or confusing steps
- No dropper bottle or number of drops required
- No batteries or instruments needed
- Designed in Bethlehem, Pennsylvania by OraSure Technologies, Inc.
- Suitable for ages 15 and up
- Bilingual instructions included
*This product has not been FDA cleared or approved; but has been authorized by FDA under an EUA; This product has been authorized only for the detection of proteins from SARS- CoV-2, not for any other viruses or pathogens; and, This product is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostics for detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Federal Food, Drug and Cosmetic Act, 21 U.S.C. § 360bbb-3(b)(1), unless the declaration is terminated or authorization is revoked sooner.